Notes on the Itaconate Shunt hypothesis of ME
This post is aimed at those who are interested in biochemistry and the details of the TCA cycle - I won't be offended if you skip it 😉
I’ve just worked my way through Ron Davis’s recent video ‘Is ME/CFS curable?’ Ron Davis is a geneticist famous for his role in mapping the human genome; when I was studying biochemistry in the early 2000’s we talked about him the way physicists might talk about Stephen Hawking or Albert Einstein. His son Whitney has very severe ME, and he has spent the past decade tirelessly working towards a cure for the condition.
I think Ron Davis leaves out some background information, which is probably obvious to him but not obvious to non-experts. I thought I’d try to fill in some of the gaps.
In the Itaconate Shunt hypothesis, the process of a person developing ME begins when something triggers innate immunity. Innate immunity basically means inflammation. Inflammation can be triggered by a viral or bacterial infection, parasite, environmental toxins, trauma from e.g. surgery or a car accident, giving birth, heat or cold, and any other number of things; pretty much anything that’s stressful or harmful to the body triggers inflammation.
What’s inflammation? It isn’t just one thing, it’s a complicated set of processes. White blood cells rush to the site of inflammation, change their form and function, and rapidly multiply. Complicated sequences of chemical reactions take place. If inflammation happens on the surface of the body it looks red and swollen and feels tender; if your throat is inflamed it feels sore; but generally if there’s inflammation inside your body you won’t be aware of it.
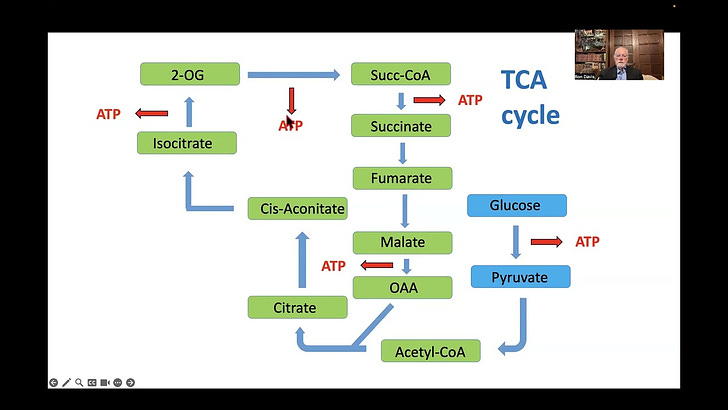
The Itaconate Shunt hypothesis involves the TCA cycle and ATP, so I should briefly explain what those are. ATP is the energy currency of cells; just like your phone needs a charger and a car needs petrol, a cell needs ATP in order to do its thing. The TCA cycle is a set of chemical reactions in the mitochondria of cells which results in the production of ATP. Confusingly the TCA cyle has several names: Tricarboxylic Acid cycle, Citric Acid cycle, and Krebs cycle.
So back to the hypothesis. Something happens to trigger inflammation. As part of the inflammatory response a chemical called interferon alpha, often written IFN-α, is released into the blood. IFN-α has a complicated set of effects; one of them is that it (and I’m not clear on how) causes an enzyme called cis-aconitate decarboxylase (CAD) to be produced in cells. CAD travels to the mitochondria, where it disrupts the TCA cycle and prevents the cell from producing ATP (I’ll explain how this works in excrutiating detail further on).
In the normal course of things all of this happens only for a short time, after which the inflammatory response dies down, CAD stops being expressed, and things go back to normal. But (according to the hypothesis) in people with ME the body gets stuck continually producing CAD, so that the TCA cycle is continuously disrupted.
Why does this happen? One possibility, Davis says, is a vicious cycle: inflammation disrupts the TCA cycle, preventing it from aerobically making ATP; this situation in turn produces more inflammation.
[One of the problems with the TCA cycle, and biochemistry in general, is that there are too many things with confusing names; from here on in I’ll put metabolites in bold and enzymes in italics, although I don’t know if it’ll really help.]
I was confused the first couple of times I watched the video because I had never heard of the molecule cis-aconitate, which features prominently. I had to do a bit of web-searching to realise that cis-aconitate is in fact part of the TCA cycle, but it’s a molecule that only exists momentarily - as soon as it is created it immediately gets turned into something else - so it is usually left out of diagrams in textbooks. My old biochemistry textbook shows citrate being converted directly to isocitrate. This isn’t quite right; what actually happens is that citrate is converted to cis-aconitate, which only exists momentarily before being immediately converted to iso-citrate.

Remember cis-aconitate decarboxylase (CAD) the enzyme produced when there is inflammation? As far as I can tell, what it does is: it goes to the mitochondria and grabs cis-aconitate - which, remember, only exists for a moment - away from the enzyme aconitase (which was about to turn it into isocitrate) and turns it instead into itaconate. The itaconate then goes through a few more steps and ends up being converted to pyruvate, which starts the TCA cycle again from the begining. This is called a shunt because it skips the part of the TCA cycle that would result in the production of ATP.
The enzyme cis-aconite decarboxylase and the gene ACOD1 which encodes it seem to be a hot topic of research at the moment - it seems likely that these are widely important in immunometabolism and disease, not just in ME. This gives me hope that there will be a lot of research and new discoveries to come in the next few years.
This video helped me understand, in way too much detail, what the enzyme aconitase does:


Thank you for taking the time. That explanation is helpful.
Another couple aspects to this dysfunction is it may be a normal reaction to ongoing virus. It may also be related to channelopathies. The research at Griffith University (Sonya Marshall-Gradisnik) showing TRPM channelopathies is another finding that might impact this research.