The science of amyloid microclots in Long Covid and ME/CFS
Back in October of 2021, Dr Etheresia Pretorius published her discovery that the blood of people with Long Covid contains unusual clots, known as amyloid microclots or fibrinaloids. These microscopic clots are too small to cause the conditions we normally associate with blood clots, such as stroke or pulmonary embolism. But they are large enough block capillaries, the tiny blood vessels where oxygen is transferred from the blood to the body’s tissues. And, because of their unusual chemical characteristics, microclots are harder for the body to get rid of than regular blood clots. Some researchers believe that microclots could be the main cause of the symptoms of Long Covid. Interestingly, some but not all ME/CFS sufferers have microclots as well.
Before taking a dive into the science of microclots, we need to take a look at how blood clotting works in general.
Blood clotting
Our bodies contain a network of blood vessels, many of them much smaller than a human hair, ensuring that blood is delivered to every part of the body. Blood clotting is the body’s way of making sure that, if a blood vessel is cut open, we don’t bleed out.
Blood clotting is a rather complicated, multi-step process. It starts when a blood vessel wall is damaged. The walls of blood vessels contain collagen, and when a blood vessel is cut open the collagen becomes exposed.

Blood contains cells (strictly speaking, cell fragments, since they have no nucleus) called platelets. In their inactive form platelets have a flat shape rather like a plate, hence the name. But when platelets come into contact with collagen from a damaged blood vessel wall, they become activated. They change shape, growing long ‘arms’, and become sticky, so that they can attach themselves to the collagen, and to each-other. Within 30 to 60 seconds the activated platelets form a sticky plug over the damaged blood vessel wall. It isn’t very strong, but it’s enough to stop or slow the bleeding.

Activated platelets also release chemicals called clotting factors. Meanwhile, the blood contains a lot of a dissolved protein called fibrinogen. Most of the time fibrinogen doesn’t do anything at all, it’s just kind of there. But the clotting factors transform the fibrinogen into fibrin, which forms long, sticky, insoluble strands that attach to the platelet plug and form a clot. The clot remains until the wound heals, then falls off or is broken down.
Hypercoagulability and dangerous blood clots
Our blood contains a balance of chemicals that promote clotting - coagulants - and chemicals that dissolve clots and inhibit clotting - anti-coagulants. When we get a cut, clotting factors are released which temporarily tip this balance in favour of clotting, but this only happens at the site of the cut, and it only lasts as long as it takes for a clot to form and close the wound.
In general, it’s very dangerous for the balance between coagulants and anti-coagulants to be disturbed, in either direction.
Hemophilia is an inherited disease where the body doesn’t produce enough clotting factors, and as a result it’s more difficult for the body to produce blood clots. Hemophiliacs can continue bleeding for a long time, even after a minor cut.
On the other hand some people have blood that is hypercoagulable, that is to say, it clots too easily. Experts aren’t sure precisely why this happens, but they have some ideas. The risk factors for hypercoagulable blood include smoking, obesity, diabetes, and age, all of which are associated with systemic inflammation, so it may be that inflammation is what causes the blood to become hypercoagulable.
Hypercoagulable blood is dangerous because a clot could form in a blood vessel even when there is no wound to heal. Hypercoagulable blood is found in people with acute Covid, and research by Etheresia Pretorius and her team shows that people with Long Covid have somewhat hypercoagulable blood as well.
Microclots are different (or, a very brief introduction to the three-dimensional shapes of proteins)
Amyloid microclots are different from regular blood clots. What makes them different? This gets a little complicated, and involves some chemistry.
Let’s start with the question: “What are amyloid microclots made of?” My instinct when I first heard the term was to assume they’re made of ‘amyloid’, whatever that is, but it turns out ‘amyloid’ is an adjective, not a noun. Amyloid microclots, like regular blood clots, contain a lot of fibrin. But not regular fibrin - amyloid fibrin.
Fibrin, like many important biological molecules, is a protein. A protein molecule is made up of a long chain of amino acids - and yet the three dimensional shape of most proteins isn’t a long chain, it’s generally some sort of three-dimensional blob. Each protein has a very particular shape, and the shape is very important for that protein’s function. If a protein takes the wrong shape it loses its ability to function properly.
To understand the shapes of proteins, we need to know that proteins have three levels of structure (actually four, but we’ll ignore the last one).
The primary structure of a protein is its sequence of amino acids, as you’d expect.
There are two options for a protein’s secondary structure. The chain of amino acids can form a helix shape, like a single strand of DNA - this is called an alpha-helix. Alternatively, it can form a zig-zag shape, with multiple zig-zagging amino acid chains lined up parallel or anti-parallel to each-other - this structure is called a beta-sheet. Either way, the secondary structure is determined by hydrogen bonds which form between nearby amino acids.
The tertiary structure is determined by the way the alpha-helix or beta-sheet folds in on itself. There are infinite possibilities, and the tertiary structure is really what makes a protein unique. It is determined by hydrogen bonds and van der Waals forces between different amino acids within the protein, and between the amino acids and the water that the protein is surrounded by.
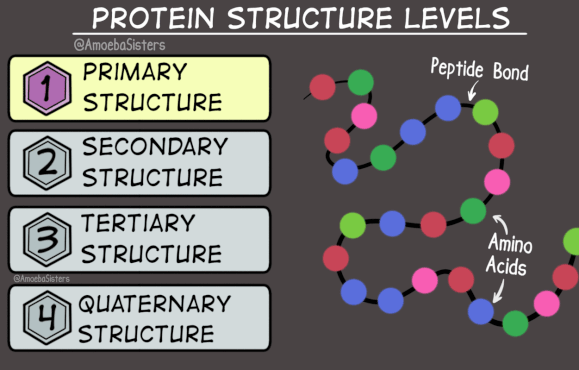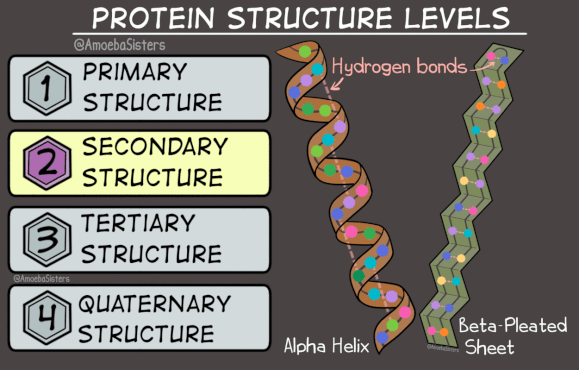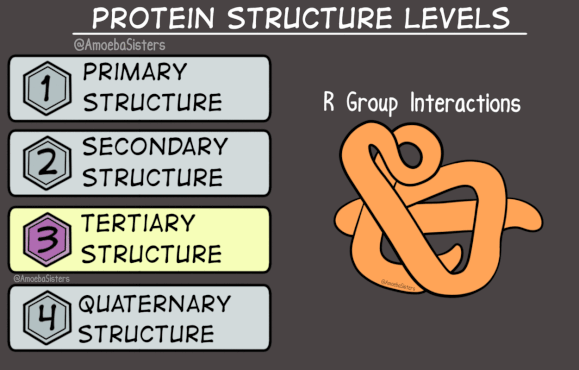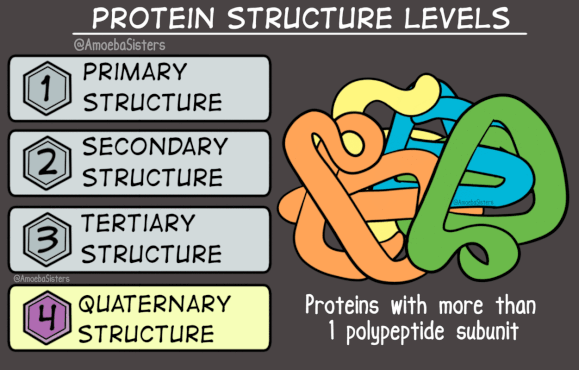
Here’s where it gets weird. There’s an alternate three dimensional structure that any protein can take: it consists of beta-sheets wound together to form insoluble fibres. It’s rare, and when it happens in the body it’s often a sign of disease. Proteins that take this alternate three-dimensional structure are called ‘amyloid’.
Since proteins get their properties from their three-dimensional shape, all amyloid proteins behave pretty much the same way: they stick together tightly and form insoluble clumps that are hard to dissolve. And importantly, as we’ll see, they bind to a particular type of fluorescent dye.
So what’s in amyloid microclots? There’s amyloid fibrin, and also amyloid fibrinogen (regular fibrinogen dissolves in blood, but amyloid fibrinogen, like any amyloid protein, doesn’t dissolve and forms solid clumps instead). There’s also a lot of platelets and some other blood cells trapped in there, as you’d expect to find in any blood clot.
There’s also a molecule that promotes clot formation and prevents clots from being broken down: alpha (2)-antiplasmin; and an inflammatory molecule: serum amyloid A.
Some history leading to the discovery of microclots in Long Covid
Dr Etheresia Pretorius is a researcher focusing on blood coagulation (clotting) and inflammatory diseases such as diabetes and rheumatoid arthritis, and more recently, Covid and Long Covid. Since around 2011 she’s studied ‘dense matted deposits’ in the blood of people with diabetes, and in 2017 her group found that these deposits are amyloid in nature. Microclots aren’t unique to Long Covid, they’re also found in the blood of people with diabetes, although people with Long Covid have them in greater amounts.
Detecting amyloid microclots
Microclots are tiny; their size ranges from 1 to 200 µm - that’s micrometers or millionths of a meter. They can only be seen using a powerful microscope. In order to make the microclots visible they are stained with a fluorescent dye that binds to amyloid proteins but not to anything else. You pour the dye over the sample, let it sit for a minute, wash it away, and then look at the sample under the microscope - whatever glows, is amyloid protein. (I’m oversimplifying a rather complicated procedure, but that’s the gist.)
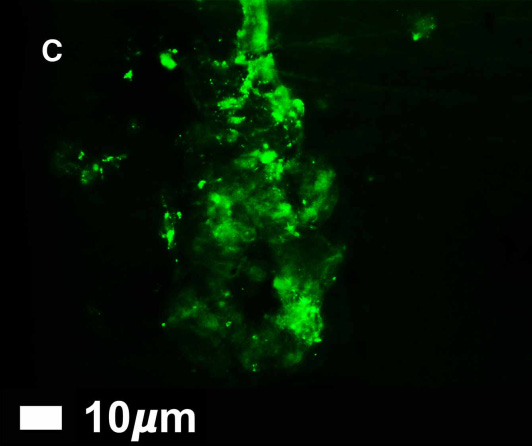
Currently only a few labs in the world are set up to detect microclots. Dr Pretorius and her colleagues are working on developing a relatively low-cost test for microclots that could be made widely available.
What causes amyloid microclots?
Researchers aren’t sure yet. There are several substances that could potentially cause protein to switch to the amyloid form. It could be the inflammatory molecule serum amyloid A, it could be the spike protein of the Covid-19 virus, it could be lipopolysaccharides from bacteria living in the body, or iron ions, estrogen, or something else.
How microclots could cause the symptoms of Long Covid and ME/CFS
Microclots are not large enough to block the body’s large blood vessels, the veins and arteries, and therefore they can’t cause the types of problems that we normally associate with blood clots: deep vein thrombosis, pulmonary embolism, heart attack, and stroke. But they could cause other problems.
Every cell of the body (with a few exceptions) needs a constant supply of oxygen - cells that are deprived of oxygen lose their ability to function and die.
Oxygen is delivered to cells through capillaries - tiny blood vessels where oxygen and nutrients move out of the blood and into cells. Capillaries are 5 to 10 µm wide, while microclots are 1 to 200 µm wide, so it would definitely be possible for microclots to block capillaries and slow the flow of blood, so that insufficient oxygen would be delivered to cells. This could cause fatigue, and microclots in the brain could explain the cognitive impairment seen in Long Covid as well.
On the other hand, while microclots could explain the symptoms of Long Covid, they aren’t the only possible explanation. It’s isn’t clear yet whether microclots are a cause of Long Covid, or are caused by it, or both.
The fact that some people with ME/CFS have microclots while others don’t is puzzling. One possible explanation is that ME/CFS may have sub-types, where different underlying processes could lead to the same disease. Another possibility is that microclots appear only in the earlier stages of the disease; Long Covid sufferers have been ill for less than three years, while many ME/CFS sufferers have been ill for far longer.
Further reading - for a general audience
Could microclots help explain the mystery of long Covid? The Guardian
Inflammatory micro clots in blood of individuals suffering from Long COVID, ScienceDaily
Could tiny blood clots cause long COVID’s puzzling symptoms? Nature News feature
Develop an accessible, lab-based method to diagnose microclots in LongCovid patients - fundraising page
Misfolded spike protein could explain complicated COVID-19 symptoms, MedicalNewsToday
Further reading - scientific articles
Serum amyloid A binds to fibrin(ogen), promoting fibrin amyloid formation, 2019
A new era for understanding amyloid structures and disease, 2018